Safety and efficacy of oxaliplatin-based chemotherapy in the first line treatment of elderly patients affected by metastatic colorectal cancer
WCRJ 2014; 1 (2) : e235
Topic: Geriatric oncology
Category: Original article
Abstract
INTRODUCTION: Elderly patients constitute a subpopulation with special clinical features that differ from those of the general population and are under-represented in clinical trials.
MATERIALS AND METHODS: We analyzed the toxicity and efficacy of an oxaliplatin-based chemotherapy (FOLFOX2, FOLFOX4 and XELOX) in the treatment of elderly patients affected by metastatic (m) colorectal cancer (CRC). One hundred and sixty-seven consecutive patients (FOLFOX2 20 patients; FOLFOX4 36 patients; XELOX 111 patients) aged 65 to 85 years (median age 75 years), 101 males and 66 females, with mCRC and measurable disease, were analyzed. The primary site of metastases was the liver (44% of patients). The majority of patients had a median performance status (PS) (ECOG) of 0 (range 0-2).
RESULTS: The overall response rates according to the treatment schedules were: FOLFOX2 55%, FOLFOX4 44,4%, and XELOX 40.4%. The median progression-free survival (PFS) was about 7.3 months in all treatments and the median overall survival (OS) rates were: FOLFOX 2 21.8 months, FOLFOX4 16 months and XELOX 16 months. The main hematological and extra-hematological toxicities (grade 3 or 4) were neutropenia (14.4%), and neurological toxicity or diarrhea (15%). No toxic death occurred.
CONCLUSIONS: Oxaliplatin-based chemotherapy maintain its efficacy, and safety in elderly patients with mCRC and good PS. The different results in terms of PFS and OS, according to the treatment performed, could be dependent on the different number of patients enrolled in each study. This regimen should be considered in the treatment of this particular setting of patients.
INTRODUCTION
Colorectal cancer (CRC) is one of the most common malignancies and the second cause of cancer death in the US and most European countries. Its incidence has been increasing in the last decades, primarily as a consequence of the aging of the population. In Europe more than 40% of new CRCs are diagnosed in patients older than 75 years 1 , 2.
With an increasing number of elderly patients likely to be diagnosed with CRC in the upcoming decades, it is of interest how this population tolerates and responds to modern chemotherapy regimens 3.
In spite of these demographics, little is known about the impact of age on the morbidity of cancer treatment in elderly patients 4.
Standard treatments for mCRC include palliative chemotherapy, with an expanding range of available options, but the evidence supporting these treatments derives from clinical trials where elderly or frail patients are under-represented 5 , 6 , 7.
Several pivotal trials were restricted to patients younger than 75 years 8. However, even when a formal upper age limit was not an inclusion criterion, the recruitment of elderly patients was difficult, and the few included were highly selected 9.
Noteworthy, elderly patients are characterized by frequent incidence of age-related co-morbidities such as impaired renal, cardiac, and liver function, general decline in health, loss of autonomy, and cognitive impairment that may impact on the therapeutic decision 10 , 11.
Nevertheless, the treatment of mCRC in elderly patients is still a challenge, the overall therapeutic strategy in this population should be individualized, and a general consensus on how to treat elderly patients with mCRC is still far from being achieved. Given the great importance of elderly population with CRC, it is central to systematically assess the management of elderly CRC patients with modern chemotherapeutic regimens. Fortunately, in the recent period more attention has been dedicated to this particular setting of patients and it is notable in the English literature 12 , 13 , 14 , 15 , 16.
Oxaliplatin- or irinotecan-based combinations have increased the treatment options for patients with mCRC. Various phase III trials showed improved progression-free survival (PFS), RRs, and overall survival (OS) when infusional 5-FU/LV was combined with oxaliplatin or irinotecan compared with 5-FU/LV alone 17 , 18 , 19. More recently, oxaliplatin-based combinations were shown useful and safe in selected elderly patients with mCRC 20 , 21 , 22 , 23 , 24 , 25 , 26.
In particular, oxaliplatin and 5-FU have a synergistic activity both in vitro and in vivo studies against colon cancer cells 27 , 28 22,23. In recent years, several regimens with oxaliplatin in combination with leucovorin and 5-FU in continuous infusion have been developed 29 , 30 , 31 , 32, such as FOLFOX-2, FOLFOX-4 and XELOX 33.
The overall results suggest at least a similar activity in older patients, in comparison to the general population, though there are conflicting results regarding the toxicities in elderly patients. In particular, hematologic and oxaliplatin-induced neurotoxicity (particularly among diabetics) are of main concern 34 , 35 , 36.
We report our experience on the use of chemotherapy in elderly patients with mCRC. In particular, in this study we explore feasibility and safety of oxaliplatin-based chemotherapy in our cohort of mCRC elderly patients reporting data on treatment response, toxicity and survival.
PATIENTS AND METHODS
Patients selection
From March 1993 to December 2010, 167 consecutive patients affected by mCRC (histologically confirmed), with adequate organ functions (defined as less than twice the upper normal values of internal ranges), absence of major chronic diseases, bi-dimensionally measurable metastases evaluated by Computed Tomography (CT) scans) and ECOG PS ≤ 2, were considered eligible for this study.
Treatment schedule
These different kinds of treatment were: FOLFOX2, FOLFOX4 and XELOX.
The FOLFOX2 regimen comprised oxaliplatin 100 mg/m2 as a 2-hour infusion on day 1, leucovorin 500 mg/m2 as a 2-hour infusion on days 1 and 2, followed by 5-fluorouracil 1.5 g/m2 as 22-hour infusion for two consecutive days; every 2 weeks.
FOLFOX4 regimen comprised leucovorin 200 mg/m2/day in a 2-hour infusion, followed by bolus 5-fluorouracil 400 mg/m2/day and 5-fluorouracil (600 mg/m2/day in a 22-hour infusion) day 1 and 2 every 2 weeks, plus oxaliplatin 85 mg/m2 in a 2-hour infusion without prior mixing, on day 1. The cycles are repeated at a 2-week interval.
The XELOX regimen comprised oxaliplatin 130 mg/m2 day 1 then oral capecitabine 1,000 mg/m2 twice a day, from the evening of day 1 to the morning of day 15, followed by a 7-day treatment-free interval, in a 3-week cycle.
Capecitabine starting dose was reduced to 75% in patients with moderate renal impairment (30 mL/min ≤ creatinine clearance < 50 mL/min), and adjusted for adverse events of grade (G) 2 or of higher intensity, according to the literature 37. Oxaliplatin dose was reduced for severe vomiting, G3 or 4 thrombocytopenia, for G4 neutropenia, or for significant neurological toxicity. In case paresthesiae with functional impairment persistent between cycles, oxaliplatin was discontinued. The planned number of treatment cycles was 6; patients maintaining response or stable disease after the planned cycles could further continue treatment with the same regimen or with capecitabine alone. Also in the XELOX regimen, the patients could continue capecitabine mono-therapy after discontinuation of oxaliplatin for neurotoxicity, regardless the number of received cycles.
Treatment was maintained until either disease progressed or unacceptable toxicity appeared. Patients received antiemetic prophylaxis as routine practice of each participating center 38. The prophylactic use of colony-stimulating factors was not allowed 39.
The treatment was reduced to 75% of the calculated dose when hematological toxicity greater than G3 occurred.
Evaluation during the study
At baseline, patients underwent a clinical history and physical examination, blood counts, liver and kidney function tests and evaluation of electrolyte concentrations, and prothrombine time. ECG, and CT-scan of the abdomen and thorax were performed before treatment start.
During the treatment, blood counts were performed on day 7 of the first two cycles, and then at the beginning of each following cycle, together with blood chemistry. Tumor response studies were performed every 6 cycles or earlier in case of clinical deterioration.
Safety and toxicity
Tumor response was evaluated by investigators according to the response evaluation criteria in solid tumors (RECIST) 40 at 3-month intervals until the disease progression or patient death. Toxicity was evaluated at the beginning of each cycle using the National Cancer Institute Common Toxicity criteria scale, version 2.0.
Statistical analysis
The PFS and OS times were calculated from the start of treatment until evidence of disease progression or death, respectively.
Data on response rates are expressed as the proportion of responders (complete response and partial response) in relation to all the other categories (stable disease, progressive disease and not classified).
Survival analyses were calculated according to Kaplan-Meier method 41 and differences between subgroups were assessed by means of the log-rank test 42. In all cases, statistical significance was claimed as p < 0.05 (two sided) 43.
RESULTS
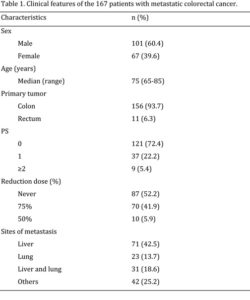
Between March 1993 and December 2010, 167 patients were retrospectively evaluated. All patients were assessable for toxicity and antitumoral activity. Baseline patient characteristics are listed in Table 1. The majority of patients were male (60.4%). Median patient age was 75 years (range 65-85). Most patients had a median ECOG PS before treatment of 0 (range 0-2) (72.4%), and more than half had only one metastatic location. Liver was interested by metastases in 42.5% of the patients, lung in 13.7% of the patients, liver and lung, together, in 18.5% of the patients. Twenty-one percent of the patients had received adjuvant chemotherapy with fluorouracil plus leucovorin or oral fluoropyrimidines.
Treatment compliance
A total of 1250 chemotherapy courses were administered (332 FOLFOX2, 334 FOLFOX4 and 584 XELOX). Seventy patients (22.7%) and 5 patients, received 75% and 50%, respectively (14 and 53 patients in the FOLFOx4 and XELOX treatment, respectively, received 75% of dose, while 1 and 5 patients in the FOLFOX2 and XELOX regimens, respectively, received the 50% dose schedule).
Safety
The hematological and non-hematological toxicities of the patients are listed in Table 2. The main hematological toxicity was grade 3-4 neutropenia in 14.4% of patients. Among non-hematological toxicities neurological toxicity and diarrhea were the more frequent with grade 3-4 occurring in 8.4% and 6.6% of patients, respectively. Dysphonia was reported in 29 patients (17.3%) 44. No deaths due to toxicity occurred.
Response to treatment
The 167 patients included in the study were considered assessable for response. Complete response was achieved in 18 patients (10.7%), and partial response was achieved in 66 patients (39.5%) for a total overall response rate of 50.2%. Disease response was assessed by CT scan after six cycles. Forty-seven (28.1%) patients achieved disease stabilization. Consequently, 78.3% of all patients included in the study obtained disease control.
Survival analysis
Figures 1 and 2 show the overall survival for FOLFOX2, FOLFOX4 regimens. After a median follow-up of 27 months (1-124 months) at the time of analysis, the median PFS was 7.3 months (range 1-30), and the median OS time was 22 months (range 1-124).
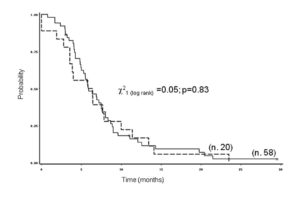
Figure 1. Time to progression of 78 patients with metastatic colorectal cancer by age at diagnosis (__middle aged: < 70 vs. — elderly: ≥ 70 years).
Prognostic factors
Analyses of prognostic factors were studied in XELOX regimen, initial ECOG PS (0+1 vs. >2), histological sub-type (mucinosus vs. non-mucinosus), number and site of metastases (1 metastasis vs. 2 or more metastases and lung+liver vs. other), CEA value (normal vs. 2/3-fold normal value), comorbidities (no comorbidities vs. comorbidities), dose chemotherapy reduction (reduction vs. non-reduction) and age (75 years or more vs. more than 75 years old). No predictive factors for response were found. The same variables were analyzed for OS (Figure 3) and PFS (Figure 4), also including the response to treatment (CR or PR vs. SD and PD). We have found that the patients with a worse prognosis were the ones with more than one metastasis, elevated CEA value and those who have received a reduced dose schedule, verso those with one metastasis, normal CEA value and full dose schedule treatment.
DISCUSSION
In the ageing countries 45 , CRC predominantly affects older people and produces a soaring demand for care in those patients. Although the median age of those diagnosed with CRC exceed 70 years both in Europe and in the US 46 , 47 , elderly patients are scantly represented into clinical trials 48 , with less than 20% rate included in the key studies 49 , 50 , 51.
Chronologic age has been a major barrier for clinicians to offer the best treatment modalities to elderly population. However, chronologic age does not always correspond to real physiologic age. Aging is characterized by presence of co-morbidities such as diabetes, cardiovascular disease and by the development of physiologic changes in all organs which will affect how a chemotherapeutic agent is absorbed, metabolized and eliminated. However, increasing chronologic age does not equate to a uniform decline in physiologic reserve of all systems in all individuals. Elderly population is not homogenous in health status: some are healthy, while others are extremely frail, affected by one or more co-morbid diseases that may influence treatment tolerance. It’s important to notice that elderly patients are under-represented in clinical trials, but also that the few included share a good performance status, are highly functional and independent. Data obtained from this selected studies can’t be extended to general population, without rough approximations.
In spite of the magnitude of the problem, the treatment of CRC in elderly patients remains a challenge. Notably, elderly patients are less treated with chemotherapy, both in adjuvant and palliative setting, than general population 52. This trend may be attributed to: 1) few data on safety and feasibility of chemotherapy in CRC elderly patients that are often excluded from studies; 2) concerns about toxic effects of drugs influencing quality of life; 3) presence of multiple co-morbidities that may influence the treatment tolerance.
Palliative chemotherapy remains the mainstay of treatment for patients with non-resectable or mCRC. Systemic chemotherapy may prolong survival, decreases tumor-related symptoms, improves general wellbeing or maintains it for a longer period of time when compared with the best supportive care.
Ho et al 53 reported that the use of palliative chemotherapy for mCRC seems to decline with age; while over 70% of patients younger than 70 years receive some chemotherapy for mCRC, only 43% of patients older than 70 years receive palliative chemotherapy. This trend has been recently confirmed by the Australian Cancer Registry 54. To support the use of chemotherapy in elderly patients, Cascinu et al 55 have already demonstrated that fit elderly patients with advanced cancer are not harmed by full doses of chemotherapy.
Several studies have shown that elderly patients obtain a similar benefit than younger patients 56 , 57 , 58 , 59. Moreover, overall available data suggests that toxicity does not seem to show different patterns in patients over and under 70 years 60 , 61 , 62 , 63.
More recently, many authors have reported the safety and the efficacy of oxaliplatin-based chemotherapy in the treatment of CRC (both adjuvant and palliative setting) elderly patients (Table 3) 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71.
Initial studies 72 reported an increase of gastrointestinal toxicity in patients older than 65 years of age with FOLFOX regimen and a small but significant increase in G3-G4 neutropenia and thrombocytopenia; but this has not been confirmed by later trials 73 , 74 , 75.
In a recent SEER analysis focused on older mCRC patients exposed to oxaliplatin and not included in clinical trials, no survival differences were noted, compared to similarly aged patients exposed to FOLFOX, with fewer adverse events and overall safer toxicity profile 76.
Furthermore, a reduction in the rate of these toxicities have been achieved remaining similar efficacy 77 , 78 through several modifications in the FOLFOX regimen (fractionated oxaliplatin or dose reduction) or the association with neuroprotective agents.
In this study, we investigated activity and safety of oxaliplatin-based chemotherapy in the treatment of mCRC elderly patients. Results can summarize as follows.
First, we obtained a complete response in 18 patients (10.7%) and a partial response in 66 patients (39.5%) for a total overall response rate of 50.2%. This outcome translated in a median PFS of 7.5 months and a median overall survival time of 22 months. These results are similar to those reported in the literature (Table 3) and suggest that combination chemotherapy should not be denied to elderly patients who have been selected carefully on the basis of PS and comorbidities, and who are willing to receive curative treatment for their cancer.
Second, among analyzed prognostic factors (including sex, age, initial ECOG PS, location of metastases, number of metastases, CEA value, dose chemotherapy reduction) only the number of metastases (> 1) and the CEA value significantly influenced survival (c22 = 33.82; p < 0.0001), similarly to general population.
Third, the main G3-G4 hematological toxicity was neutropenia (24 patients, 14.4%), while G3-G4 neurological toxicity and diarrhea (non-hematological) occurred both in 25 patients only (15%). Moreover, no patient was admitted to the hospital because of toxicity and no toxic deaths occurred. Unfortunately, no data reporting cancer-related fatigue have been analyzed 79 , 80 , 81.
Fourth, it is to point out that the patients included into this study are a subpopulation of elderly patients characterized by their good PS and free from the typical geriatric syndromes. As a consequence, our data corroborating the safety and feasibility of oxaliplatin based chemotherapy in mCRC elderly patients should be extended with caution to the entire elderly population. Moreover, we hardly encourage studies including less fit and frail patients that represent a large part of elderly population affected by cancer.
CONCLUSIONS
Chronologic age should not be a limiting factor for the decision making process for patients with mCRC who are considering treatment with oxaliplatin based chemotherapy. In fact, elderly cancer patients represent a new challenge in the third millennium 82 , 83 , 84. Moreover, with the aid of pharmacogenomic tests, we can better select elderly cancer patients and related treatments 85 , 86 , 87 , 88 , 89.
However, elderly patients should be individually examined for PS, the presence or absence of comorbid medical conditions, independence in activities of daily living and carefully assessed as concerns relative risks and benefits for treatment. Our data add to those in the literature that support the use of adequate chemotherapy for elderly patients in good clinical conditions. Careful monitoring for toxicity and rapid intervention with supportive care measures when toxicity occurs is also mandatory, particularly in elderly patients.
Conflict of Interests:The Authors declare that they have no conflict of interests.
Acknowledgements
The authors thank Mrs. Paola Favetta, for her expert assistance in the preparation and correction of the manuscript.
References
- Berretta M, Zanet E, Nasti G, Lleshi A, Frustaci S, Fiorica F, Bearz A, Talamini R, Lestuzzi C, Lazzarini R, Fisichella R, Cannizzaro R, Iaffaioli RV, Berretta S, Tirelli U. Oxaliplatin-based chemotherapy in the treatment of elderly patients with metastatic colorectal cancer (CRC). Arch Gerontol Geriatr 2012; 55: 271-275. (back)
- Berretta M, Aprile G, Nasti G, Urbani M, Bearz A, Lutrino S, Foltran L, Ferrari L, Talamini R, Fiorica F, Lleshi A, Canzonieri V, Lestuzzi C, Borsatti E, Fisichella R, Tirelli U. Oxaliplapin and capecitabine (XELOX) based chemotherapy in the treatment of metastatic colorectal cancer: the right choice in elderly patients. Anticancer Agents Med Chem 2013; 13: 1344-1353. (back)
- Winawer SJ, Fletcher RH, Miller L, Godlee F, Stolar MH, Mulrow CD, Woolf SH, Glick SN, Ganiats TG, Bond JH, Rosen L, Zapka JG, Olsen SJ, Giardiello FM, Sisk JE, Van Antwerp R, Brown-Davis C, Marciniak DA, Mayer RJ. Colorectal cancer screening: clinical guidelines and rationale. Gastroenterology 1997; 112: 594-642. (back)
- Berardi R, Saladino T, Mari D, Silva RR, Scartozzi M, Verdecchia L, Onofri A, Cascinu S. Elderly patients with advanced colorectal cancer: tolerability and activity of chemotherapy. Tumori 2005; 91: 463-466. (back)
- Folprecht G, Seymour MT, Saltz L, Douillard JY, Hecker H, Stephens RJ, Maughan TS, Van Cutsem E, Rougier P, Mitry E, Schubert U, Köhne CH. Irinotecan/fluorouracil combination in first-line therapy of older and younger patients with metastatic colorectal cancer: combined analysis of 2691 patients in randomized controlled trials. J Clin Oncol 2008; 26: 1443-1451. (back)
- Hutchins LF, Unger JM, Crowley JJ, Coltman CA Jr, Albain KS. Underrepresentation of patients 65 years age or older in cancer-treatment trials. N Engl J Med 1999; 341: 2061-2067. (back)
- Seymour MT, Thompson LC, Wasan HS, Middleton G, Brewster AE, Shepherd SF, O’Mahony MS, Maughan TS, Parmar M, Langley RE. FOCUS2 Investigators; National Cancer Research Institute Colorectal Cancer Clinical Studies Group. Chemotherapy options in elderly and frail patients with metastatic colorectal cancer (MRC FOCUS 2): an open-label; randomised factorial trial. Lancet 2011; 377: 1749-1759. (back)
- Seymour MT, Thompson LC, Wasan HS, Middleton G, Brewster AE, Shepherd SF, O’Mahony MS, Maughan TS, Parmar M, Langley RE. FOCUS2 Investigators; National Cancer Research Institute Colorectal Cancer Clinical Studies Group. Chemotherapy options in elderly and frail patients with metastatic colorectal cancer (MRC FOCUS 2): an open-label; randomised factorial trial. Lancet 2011; 377: 1749-1759. (back)
- Townsley CA, Selby R, Siu LL. Systematic review of barriers to the recruitment of older patients with cancer onto clinical trials. J Clin Oncol 2005; 23: 3112-3124. (back)
- Mahoney T, Kuo YH, Topilow A, Davis JM. Stage III colon cancers: why adjuvant chemotherapy is not offered to elderly patients. Arch Surg 2000; 135: 182-185. (back)
- Extermann M, Albrand G, Chen H, Zanetta S, Schonwetter R, Zulian GB, Cantor A, Droz JP. Are older French patients as willing as older American patients to undertake chemotherapy? J Clin Oncol 2003; 21: 3214-3219. (back)
- Berretta M, Zanet E, Nasti G, Lleshi A, Frustaci S, Fiorica F, Bearz A, Talamini R, Lestuzzi C, Lazzarini R, Fisichella R, Cannizzaro R, Iaffaioli RV, Berretta S, Tirelli U. Oxaliplatin-based chemotherapy in the treatment of elderly patients with metastatic colorectal cancer (CRC). Arch Gerontol Geriatr 2012; 55: 271-275. (back)
- Berretta M, Aprile G, Nasti G, Urbani M, Bearz A, Lutrino S, Foltran L, Ferrari L, Talamini R, Fiorica F, Lleshi A, Canzonieri V, Lestuzzi C, Borsatti E, Fisichella R, Tirelli U. Oxaliplapin and capecitabine (XELOX) based chemotherapy in the treatment of metastatic colorectal cancer: the right choice in elderly patients. Anticancer Agents Med Chem 2013; 13: 1344-1353. (back)
- Fiorica F, Berretta M, Ursino S, Fisichella R, Lleshi A, Fiorica G, Stefanelli A, Zini G, Tirelli U, Zanghi A, Cappellani A, Berretta S, Cartei F. Adjuvant radiotherapy on older and oldest breast cancer patients after conservative surgery: a retrospective analysis. Arch Gerontol Geriatr 2012; 55: 283-288. (back)
- De Gramont A, Figer M, Seymour M, Homerin M, Hmissi A, Cassidy J, Boni C, Cortes-Funes H, Cervantes A, Freyer G, Papamichael D, Le Bail N, Louvet C, Hendler D, De Braud F, Wilson C, Morvan F, Bonetti A. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol 2000; 18: 2938-2947. (back)
- Douillard JY, Cunningham D, Roth AD, Navarro M, James RD, Karasek P, Jandik P, Iveson T, Carmichael J, Alakl M, Gruia G, Awad L, Rougier P. Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: a multicentre randomised trial. Lancet 2000; 355: 1041-1057. (back)
- Goldberg RM, Sargent DJ, Morton RF, Fuchs CS, Ramanathan RK, Williamson SK, Findlay BP, Pitot HC, Alberts SR. A randomised controlled trial of fluorouracil plus leucovorin, irinotecan, and oxaliplatin combinations in patients with previously untreated metastatic colorectal cancer. J Clin Oncol 2004; 22: 23-30. (back)
- Berretta M, Cappellani A, Fiorica F, Nasti G, Frustaci S, Fisichella R, Bearz A, Talamini R, Lleshi A, Tambaro R, Cocciolo A, Ristagno M, Bolognese A, Basile F, Meneguzzo N, Berretta S, Tirelli U. FOLFOX4 in the treatment of metastatic colorectal cancer in elderly patients:a prospective study. Arch Gerontol Geriatr 2011; 52: 89-93. (back)
- Berretta M, Di Benedetto F, Di Francia R, Lo Menzo E, Palmeri S, De Paoli P, Tirelli U. Colorectal cancer in elderly patients: from best supportive care to cure. Anticancer Agents Med Chem 2013; 13: 1332-1343. (back)
- De Gramont A, Figer M, Seymour M, Homerin M, Hmissi A, Cassidy J, Boni C, Cortes-Funes H, Cervantes A, Freyer G, Papamichael D, Le Bail N, Louvet C, Hendler D, De Braud F, Wilson C, Morvan F, Bonetti A. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol 2000; 18: 2938-2947. (back)
- Douillard JY, Cunningham D, Roth AD, Navarro M, James RD, Karasek P, Jandik P, Iveson T, Carmichael J, Alakl M, Gruia G, Awad L, Rougier P. Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: a multicentre randomised trial. Lancet 2000; 355: 1041-1057. (back)
- Berretta M, Bearz A, Frustaci S, Talamini R, Lombardi D, Fratino L, Lleshi A, Bonanno S, Spartà D, Palmucci S, Berretta S, Tirelli U. Folfox2 in the treatment of advanced colorectal cancer: a comparison between elderly and middle aged patients. J Chemother 2008; 20: 503-508. (back)
- Comella P, Natale D, Farris A, Gambardella A, Maiorino L, Massidda B, Casaretti R, Tafuto S, Lorusso V, Leo S, Cannone M. Capecitabine plus oxaliplatin for the first-line treatment of elderly patients with metastatic colorectal carcinoma: final results of the Southern Italy Cooperative Oncology Group Trial 0108. Cancer 2005; 104: 282-289. (back)
- Kim JH, Oh DY, Kim YJ, Han SW, Choi IS, Kim DW, Im SA, Kim TY, Lee JS, Heo DS, Bang YJ, Kim NK. Reduced dose intensity Folfox-4 as first line palliative chemotherapy in elderly patients with advanced colorectal cancer. J Korean Med Sci 2005; 20: 806-810. (back)
- Sastre J, Aranda E, Masutti B, Tabernero J, Chaves M, Abad A, Carrato A, Reina JJ, Queralt B, Gomez-Esapna A, Gonzalez-Flores E, Rivera F, Losa F, Garcia T, Sanchez-Rovira P, Maestu I, Diaz-Rubio E. Elderly patients with advanced colorectal cancer derive similar benefit without excessive toxicity after first-line chemotherapy with oxaliplatin-based combinations: comparative outcomes from the 03-TTD-01 phase III study. Crit Rev Oncol Hematol 2009; 70: 134-144. (back)
- Goldberg RM, Tabah-Fisch I, Bleiberg H, De Gramont A, Tournigand C, Andre T, Rothenberg ML, Green E, Sargent DJ. Pooled analysis of safety and efficacy of oxaliplatin plus fluorouracil/leucovorin administered bimonthly in elderly patients with colorectal cancer. J Clin Oncol 2006; 24: 4085-4091. (back)
- De Gramont A, Vignou, J, Tournigand C, Louvet C, Andre T, Varette C, Raymond E, Moreau S, Le Bail N, Krulik M. Oxaliplatin with high-dose leucovorin and 5-fluorouracil 48-hour continuous infusion in pretreated metastatic colorectal cancer. Eur J Cancer 1997; 33: 214-219. (back)
- Raymond E, Chaney SG, Taamma A, Cvitkovic E. Oxaliplatin: a review of preclinical and clinical studies. Ann Oncol 1998; 9: 1053-1071. (back)
- De Gramont A, Vignou, J, Tournigand C, Louvet C, Andre T, Varette C, Raymond E, Moreau S, Le Bail N, Krulik M. Oxaliplatin with high-dose leucovorin and 5-fluorouracil 48-hour continuous infusion in pretreated metastatic colorectal cancer. Eur J Cancer 1997; 33: 214-219. (back)
- Andre T, Louvet C, Raymond E, Tournigand C, De Gramont A. Bimonthly high-dose leucovorin, 5-fluorouracil infusion and oxaliplatin (FOLFOX3) for metastatic colorectal cancer resistant to the same leucovorin and 5-fluorouracil regimen. Ann Oncol 1998; 9: 1251-1253. (back)
- Andre T, Bensmaine MA, Louvet C, Francois E, Lucas V, Desseigne F, Beerblock K, Bouché O, Carola E, Merrouche Y, Morvan F, Dupont-André G, De Gramont A. Multicenter phase II study of bimonthly high-dose leucovorin, fluorouracil infusion and oxaliplatin for metastatic colorectal cancer resistant to the same leucovorin and fluorouracil regimen. J Clin Oncol 1999; 17: 3560-3568. (back)
- Maindrault-Goebel F, Louvet C, Andre T, Carola E, Lotz JP, Molitor JL, Garcia ML, Gilles-Amar V, Izrael V, Krulik M, De Gramont A. Oxaliplatin added to the simplified bimonthly leucovorin and 5-fluorouracil regimen as second-line therapy for metastatic colorectal cancer (FOLFOX6). GERCOR. Eur J Cancer 1999; 35: 1338-1342. (back)
- Berretta M, Aprile G, Nasti G, Urbani M, Bearz A, Lutrino S, Foltran L, Ferrari L, Talamini R, Fiorica F, Lleshi A, Canzonieri V, Lestuzzi C, Borsatti E, Fisichella R, Tirelli U. Oxaliplapin and capecitabine (XELOX) based chemotherapy in the treatment of metastatic colorectal cancer: the right choice in elderly patients. Anticancer Agents Med Chem 2013; 13: 1344-1353. (back)
- Goldberg RM, Tabah-Fisch I, Bleiberg H, De Gramont A, Tournigand C, Andre T, Rothenberg ML, Green E, Sargent DJ. Pooled analysis of safety and efficacy of oxaliplatin plus fluorouracil/leucovorin administered bimonthly in elderly patients with colorectal cancer. J Clin Oncol 2006; 24: 4085-4091. (back)
- Mattioli R, Massacesi C, Recchia F, Marcucci F, Cappelletti C, Imperatori L, Pilone A, Rocchi M, Cesta A, Laici G, Bonsignori M, Lippe P. High activity and reduced neurotoxicity of bi-fractionated oxaliplatin plus 5-fluorouracil/leucovorin for elderly patients with advanced colorectal cancer.Ann Oncol2005; 16: 1147-1151. (back)
- Figer A, Perez-Staub N, Carola E, Tourningard C, Lledo G, Flesh M, Barcelo R, Cervantes A, André T, Colin P, Louvet C, De Gramont A. Folfox in patients aged between 75 and 80 years with metastatic colorectal cancer: an exploratory cohort of the OPTIMOX1 study. Cancer 2007; 110: 2666-2671. (back)
- Blum JL, Jones SE, Buzdar AU, LoRusso P, Kuter I, Vogel C, Osterwalder B, Burger HU, Brown CS, Griffin T. Multicenter phase II study of capecitabine in paclitaxel-refractory metastatic breast cancer. J Clin Oncol 1999; 17: 485-493. (back)
- Roila F. Transferring scientific evidence to oncological practice: a trial on the impact of three different implementation strategies on antiemetic prescriptions. Support Care Cancer 2004; 12: 446-453. (back)
- Rupolo M, Lleshi A, Cacopardo B, Michieli M, Berretta M. Hematopoietic growth factors support in the elderly cancer patients treated with antiblastic chemotherapy. Anticancer Agents Med Chem 2013; 13: 1438-1443. (back)
- Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, Van Oosterom AT, Christian MC, Gwyther SG. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 2000; 92: 205-216. (back)
- Kaplan EL, Meier P. Non-parametric estimation from incomplete observations. J Am Stat Assoc 1958; 53: 457-481. (back)
- Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep 1966; 50: 163-170. (back)
- Armitage P, Berry G. Statistical Methods in Medical Research. Blackwell Sc. Publication, London, 1987. (back)
- Berretta M, Taibi R, Bearz A, La Mura N, Berretta S, Tirelli U, Frustaci S. Dysphonia as an unusual toxic event of oxaliplatin-based chemotherapy. J Chemother 2004; 16: 595-598. (back)
- Edwards BK, Brown ML, Wingo PA, Howe HL, Ward E, Ries LA, Schrag D, Jamison PM, Jemal A, Wu XC, Friedman C, Harlan L, Warren J, Anderson RN, Pickle LW. Annual report to the nation on the status of cancer; 1975-2002; featuring population-based trends in cancer treatment. J Natl Cancer Inst 2005; 97: 1407-1427. (back)
- Yee KW, Pater JL, Pho L, Zee B, Siu LL. Enrollment of older patients in cancer treatment trials in Canada: Why is age a barrier? J Clin Oncol 2003; 21: 1618-1623. (back)
- Lewis JH, Kilgore ML, Goldman DP, Trimble EL, Kaplan R, Montello MJ, Housman MG, Escarce JJ. Participation of patients 65 years of age or older in cancer clinical trials. J Clin Oncol 2003; 21: 1383-1389. (back)
- Hutchins LF, Unger JM, Crowley JJ, Coltman CA Jr, Albain KS. Underrepresentation of patients 65 years age or older in cancer-treatment trials. N Engl J Med 1999; 341: 2061-2067. (back)
- Hutchins LF, Unger JM, Crowley JJ, Coltman CA Jr, Albain KS. Underrepresentation of patients 65 years age or older in cancer-treatment trials. N Engl J Med 1999; 341: 2061-2067. (back)
- Yee KW, Pater JL, Pho L, Zee B, Siu LL. Enrollment of older patients in cancer treatment trials in Canada: Why is age a barrier? J Clin Oncol 2003; 21: 1618-1623. (back)
- Lewis JH, Kilgore ML, Goldman DP, Trimble EL, Kaplan R, Montello MJ, Housman MG, Escarce JJ. Participation of patients 65 years of age or older in cancer clinical trials. J Clin Oncol 2003; 21: 1383-1389. (back)
- Schrag D, Cramer LD, Bach PB, Begg CB. Age and adjuvant chemotherapy use after surgery for stage III colon cancer. J Natl Cancer Inst 2001; 93; 850-857. (back)
- Ho C, Ng K, O’Reilly S, Gill S. Outcomes in elderly patients with advanced colorectal cancer treated with capecitabine: a population-based analysis. Clin Colorectal Cancer 2005; 5: 279-282. (back)
- Khattak MA, Townsend AR, Beeke C, Karapetis CS, Luke C, Padbury R, Maddern G, Roder D, Price TJ. Impact of age on choice of chemotherapy and outcome in advanced colorectal cancer. Eur J Cancer 2012; 48: 1293-1298. (back)
- Cascinu S, Del Ferro E, Catalano G. Toxicity and therapeutic response to chemotherapy in patients aged 70 years or older with advanced cancer. Am J Clin Oncol 1996; 19: 371-374. (back)
- Berardi R, Saladino T, Mari D, Silva RR, Scartozzi M, Verdecchia L, Onofri A, Cascinu S. Elderly patients with advanced colorectal cancer: tolerability and activity of chemotherapy. Tumori 2005; 91: 463-466. (back)
- Goldberg RM, Tabah-Fisch I, Bleiberg H, De Gramont A, Tournigand C, Andre T, Rothenberg ML, Green E, Sargent DJ. Pooled analysis of safety and efficacy of oxaliplatin plus fluorouracil/leucovorin administered bimonthly in elderly patients with colorectal cancer. J Clin Oncol 2006; 24: 4085-4091. (back)
- Power DG, Lichtman SM. Chemotherapy for the elderly patient with colorectal cancer. Cancer J 2010; 16: 241-252. (back)
- Folprecht G, Seymour MT, Saltz L, Douillard JY, Hecker H, Stephens RJ, Maughan TS, Van Cutsem E, Rougier P, Mitry E, Schubert U, Köhne CH. Irinotecan/fluorouracil combination in first-line therapy of older and younger patients with metastatic colorectal cancer: combined analysis of 2,691 patients in randomized controlled trials. J Clin Oncol 2008; 26: 1443-1451. (back)
- Townsley CA, Selby R, Siu LL. Systematic review of barriers to the recruitment of older patients with cancer onto clinical trials. J Clin Oncol 2005; 23: 3112-3124. (back)
- Comella P, Natale D, Farris A, Gambardella A, Maiorino L, Massidda B, Casaretti R, Tafuto S, Lorusso V, Leo S, Cannone M. Capecitabine plus oxaliplatin for the first-line treatment of elderly patients with metastatic colorectal carcinoma: final results of the Southern Italy Cooperative Oncology Group Trial 0108. Cancer 2005; 104: 282-289. (back)
- Feliu J, Escudero P, Llosa F, Bolaños M, Vicent JM, Yubero A, Sanz-Lacalle JJ, Lopez R, Lopez-Gómez L, Casado E, Gómez-Reina MJ, González-Baron M. Capecitabine as first-line treatment for patients older than 70 years with metastatic colorectal cancer: an oncopaz cooperative group study. J Clin Oncol 2005; 23: 3104-3111. (back)
- Sastre J, Marcuello E, Masutti B, Navarro M, Gil S, Antòn A, Abad A, Aranda E, Maruel J, Valladares M, Maestu I, Carrato A, Vincent JM, Diaz-Rubio E; Cooperative Group for the Treatment of Digestive Tumors. Irinotecan in combination with fluorouracil in a 48-hour continuos infusion as first line chemotherapy for elderly patients with metastatic colorectal cancer: a Spanish Cooperative Group for the Treatment of Digestive Tumors study. J Clin Oncol 2005; 23: 3545-3551. (back)
- De Gramont A, Figer M, Seymour M, Homerin M, Hmissi A, Cassidy J, Boni C, Cortes-Funes H, Cervantes A, Freyer G, Papamichael D, Le Bail N, Louvet C, Hendler D, De Braud F, Wilson C, Morvan F, Bonetti A. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol 2000; 18: 2938-2947. (back)
- Douillard JY, Cunningham D, Roth AD, Navarro M, James RD, Karasek P, Jandik P, Iveson T, Carmichael J, Alakl M, Gruia G, Awad L, Rougier P. Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: a multicentre randomised trial. Lancet 2000; 355: 1041-1057. (back)
- Comella P, Natale D, Farris A, Gambardella A, Maiorino L, Massidda B, Casaretti R, Tafuto S, Lorusso V, Leo S, Cannone M. Capecitabine plus oxaliplatin for the first-line treatment of elderly patients with metastatic colorectal carcinoma: final results of the Southern Italy Cooperative Oncology Group Trial 0108. Cancer 2005; 104: 282-289. (back)
- Kim JH, Oh DY, Kim YJ, Han SW, Choi IS, Kim DW, Im SA, Kim TY, Lee JS, Heo DS, Bang YJ, Kim NK. Reduced dose intensity Folfox-4 as first line palliative chemotherapy in elderly patients with advanced colorectal cancer. J Korean Med Sci 2005; 20: 806-810. (back)
- Sastre J, Aranda E, Masutti B, Tabernero J, Chaves M, Abad A, Carrato A, Reina JJ, Queralt B, Gomez-Esapna A, Gonzalez-Flores E, Rivera F, Losa F, Garcia T, Sanchez-Rovira P, Maestu I, Diaz-Rubio E. Elderly patients with advanced colorectal cancer derive similar benefit without excessive toxicity after first-line chemotherapy with oxaliplatin-based combinations: comparative outcomes from the 03-TTD-01 phase III study. Crit Rev Oncol Hematol 2009; 70: 134-144. (back)
- Goldberg RM, Tabah-Fisch I, Bleiberg H, De Gramont A, Tournigand C, Andre T, Rothenberg ML, Green E, Sargent DJ. Pooled analysis of safety and efficacy of oxaliplatin plus fluorouracil/leucovorin administered bimonthly in elderly patients with colorectal cancer. J Clin Oncol 2006; 24: 4085-4091. (back)
- Figer A, Perez-Staub N, Carola E, Tourningard C, Lledo G, Flesh M, Barcelo R, Cervantes A, André T, Colin P, Louvet C, De Gramont A. Folfox in patients aged between 75 and 80 years with metastatic colorectal cancer: an exploratory cohort of the OPTIMOX1 study. Cancer 2007; 110: 2666-2671. (back)
- Rosati G, Cordio S, Bordonaro R, Caputo G, Novello G, Reggiardo G, Manzione L. Capecitabine in combination with oxaliplatin or irinotecan in elderly patients with advanced colorectal cancer: results of a randomized phase II study. Ann Oncol 2010; 21: 781-786 (back)
- Goldberg RM, Sargent DJ, Morton RF, Fuchs CS, Ramanathan RK, Williamson SK, Findlay BP, Pitot HC, Alberts SR. A randomised controlled trial of fluorouracil plus leucovorin, irinotecan, and oxaliplatin combinations in patients with previously untreated metastatic colorectal cancer. J Clin Oncol 2004; 22: 23-30. (back)
- Kim JH, Oh DY, Kim YJ, Han SW, Choi IS, Kim DW, Im SA, Kim TY, Lee JS, Heo DS, Bang YJ, Kim NK. Reduced dose intensity Folfox-4 as first line palliative chemotherapy in elderly patients with advanced colorectal cancer. J Korean Med Sci 2005; 20: 806-810. (back)
- Sastre J, Aranda E, Masutti B, Tabernero J, Chaves M, Abad A, Carrato A, Reina JJ, Queralt B, Gomez-Esapna A, Gonzalez-Flores E, Rivera F, Losa F, Garcia T, Sanchez-Rovira P, Maestu I, Diaz-Rubio E. Elderly patients with advanced colorectal cancer derive similar benefit without excessive toxicity after first-line chemotherapy with oxaliplatin-based combinations: comparative outcomes from the 03-TTD-01 phase III study. Crit Rev Oncol Hematol 2009; 70: 134-144. (back)
- Figer A, Perez-Staub N, Carola E, Tourningard C, Lledo G, Flesh M, Barcelo R, Cervantes A, André T, Colin P, Louvet C, De Gramont A. Folfox in patients aged between 75 and 80 years with metastatic colorectal cancer: an exploratory cohort of the OPTIMOX1 study. Cancer 2007; 110: 2666-2671. (back)
- Satram-Hoang S, Ramanan D, Lee LF, Yu S, Reyes CM, McKenna E. Comparative effectiveness of chemotherapy in elderly patients with metastatic colorectal cancer. J Clin Oncol 2012; 30(Suppl. 4): abstract 463. (back)
- Mattioli R, Massacesi C, Recchia F, Marcucci F, Cappelletti C, Imperatori L, Pilone A, Rocchi M, Cesta A, Laici G, Bonsignori M, Lippe P. High activity and reduced neurotoxicity of bi-fractionated oxaliplatin plus 5-fluorouracil/leucovorin for elderly patients with advanced colorectal cancer.Ann Oncol2005; 16: 1147-1151. (back)
- Tournigand C, Cervantes A, Figer A, Lledo G, Flesch M, Buyse M, Mineur L, Carola E, Etienne PL, Rivera F, Chirivella I, Perez-Staub N, Louvet C, André T, Tabah-Fisch I, de Gramont A. OPTIMOX1: a randomized study of FOLFOX4 or FOLFOX7 with oxaliplatin in a stop-and-Go fashion in advanced colorectal cancer–a GERCOR study.J Clin Oncol 2006; 24: 394-400. (back)
- Giacalone A, Quitadamo D, Zanet E, Berretta M, Spina M, Tirelli U. Cancer-related fatigue in the elderly. Support Care Cancer 2013; 21: 2899-2911. (back)
- Giacalone A, Berretta M, Spina M, Tirelli U. Is long-term fatigue in patients with cancer an infrequent symptom? J Clin Oncol 2012; 30: 4175. (back)
- Giacalone A, Spina M, Berretta M, Tirelli U. Two types of fatigue in cancer patients. Br J Cancer 2012; 106: 424. (back)
- Berretta M, Di Francia R, Tirelli U. Editorial – The new oncologic challenges in the 3RD millennium. WCRJ 2014; 1: e133. (back)
- Berretta M, Di Benedetto F, Di Francia R, Lo Menzo E, Palmeri S, De Paoli P, Tirelli U. Colorectal cancer in elderly patients: from best supportive care to cure. Anticancer Agents Med Chem 2013; 13: 1332-1343. (back)
- Di Francia R, Siesto RS, Valente D, Del Buono A, Pugliese S, Cecere S, Cavaliere C, Nasti G, Facchini G, Berretta M. Current strategies to minimize toxicity of oxaliplatin: selection of pharmacogenomic panel tests. Anticancer Drugs 2013; 24: 1069-1078. (back)
- Di Francia R, Siesto RS, Valente D, Spart D, Berretta M. Pharmacogenomics panel test for prevention toxicity in patient who receive Fluoropirimidine/Oxaliplatin-based therapy. Eur Rev Med Pharmacol Sci 2012; 16: 1211-1217. (back)
- Di Francia R, Valente D, Catapano O, Rupolo M, Tirelli U, Berretta M. Knowledge and skills needs for health professions about pharmacogenomics testing field. Eur Rev Med Pharmacol Sci 2012; 16: 781-788. (back)
- Di Francia R, Cimino L, Berretta M. Genetic variants influencing fluoropyrimidine based-therapy and available methods to detect them. Eur Rev Med Pharmacol Sci 2012; 16: 285-298. (back)
- Di Francia R, Berretta M, Catapano O, Canzoniero LM, Formisano L. Molecular diagnostics for pharmacogenomic testing of fluoropyrimidine based-therapy: costs, methods and applications. Clin Chem Lab Med 2011; 49: 1105-1111. (back)
- Di Francia R, Frigeri F, Berretta M, Cecchin E, Orlando C, Pinto A, Pinzani P. Decision criteria for rational selection of homogeneous genotyping platforms for pharmacogenomics testing in clinical diagnostics. Clin Chem Lab Med 2010; 48: 447-459. (back)
To cite this article
Safety and efficacy of oxaliplatin-based chemotherapy in the first line treatment of elderly patients affected by metastatic colorectal cancer
WCRJ 2014; 1 (2) : e235
Publication History
Published online: 01 Jul 2014

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.